
Richard Procunier, 29, is the owner of Northern Shore Pharmacy in North Bay, Ont. He learned about the field of pharmacogenomics in his program at the University of Toronto and resolved to bring it back to the community where he grew up. The pharmacy is now one of the first in Canada to offer pharmacogenetic testing for clients.Fred Lum/The Globe and Mail
The future arrived in North Bay on a rainy day last summer. There, on the shores of Lake Nipissing, the genomic age stepped into Richard Procunier’s drugstore.
As the birthplace of the Dionne quintuplets, the region has a history of celebrating the marvels of DNA, and Mr. Procunier threw a party. The deputy mayor attended. There were doughnuts and balloons, white, red, and blue, twisted into the shape of the double helix. They hung behind Mr. Procunier as he told the crowd that precision medicine had arrived at the Northern Shores Pharmacy with the debut of a special genetic test that would now be available for customers to purchase.
It isn’t the type of test that reveals if someone descends from Charlemagne, or has a high risk of obesity or baldness. Rather, he said, this test analyzes certain genes to try to predict if the medications a person takes will be safe and effective. People can react quite differently to the same drug, he explained, and this can lead to side effects in some, and in others, no effect whatsoever. “Take codeine,” he told the North Bay Nugget. “Some people come in with a prescription for Tylenol 3s, and some get a bang for their buck, while for others, it doesn’t work at all.” But with the new test, he said, there’s potential for people to avoid taking the wrong drug or the wrong dose – by using their DNA as a guide.
Science still has a staggering amount to learn about the workings of human DNA. Yet the specific genes that shape a person’s response to drugs are a remarkable exception – they’re well understood. Long before it was even possible to unravel the helix, researchers had sussed out the inherited biochemistry that explains why people metabolize drugs differently. And now that unravelling DNA is not only possible, but commonplace, pinpointing the genes that impact drug reactions has become one of medicine’s hottest areas.
The field – an unwieldy mouthful known as pharmacogenomics – is seen as the ticket to “personalized medicine,” health care’s dream destination, where drugs can be matched to an individual’s unique genome. Like almost everything ‘omic,’ the field is a work in progress. But ever since reading DNA has become relatively cheap and speedy, direct-to-consumer tests that assess people’s drug-response genes are taking off.
And it’s not rocket science to fathom why. Adverse drug reactions kill more than 10,000 Canadians every year, put about 200,000 people in the hospital and cost the health system an estimated $13-billion. Experts believe that pharmacogenetic testing, if universally available, could cut that tragic toll by as much as a third. In Canada’s Personal Genome Project, an ongoing effort to build an open database of whole genomes for research, nearly a quarter of the first 56 participants were found to carry genes that put them at risk of life-threatening reactions to certain drugs. Project leader Stephen Scherer, senior scientist with the University of Toronto and the Hospital for Sick Children, calls pharmacogenetics “the first big deliverable of genome sequencing that should impact the masses.”

Researchers across the country are gathering evidence to push doctors to catch up to pharmacogenomics, especially when it comes to mental-health issues where pharmacogenetic testing is the only biological way to see whether a drug might be safe or effective.Fred Lum/The Globe and Mail
When the 29-year-old Mr. Procunier learned about the field in his pharmacology program at the University of Toronto, he was determined to bring its angst-busting potential back to the community where he grew up. “People are afraid to take medications,” he says, “because of side effects.”
So while consumers grab the DNA reins – ordering pharmacogenetic test kits online and buying them over the counter in North Bay and at a growing number of other drugstores across the country – questions are mounting about why gene-guided prescribing has not become a regular part of publicly funded health care.
After all, for years pharmacogenetic tests have been a standard perk in many private health plans, available to company executives and the well heeled to help inform their medication choices. The country’s three largest insurance companies are so sure of its upsides that they’ve all launched studies to see how much they, and employers, can potentially save in disability claims: Their expectation is that gene-guided prescribing will get people on safe and effective drugs sooner, and back to work faster.
In the United States, where adverse drug reactions are the fourth-leading cause of death, the Food and Drug Administration has decided to include information about the genetic traits that may affect a person’s drug response on the safety labels of about 300 medications. Some of those, including certain painkillers, antibiotics and drugs for high blood pressure, are no strangers to the household medicine cabinet.
Meanwhile, across Canada, researchers are amassing evidence to push the outpaced public-health system to catch up, especially when it comes to treatments for mental-health conditions. Apart from this type of gene testing, there’s no biological way to gauge if a drug for depression, say, or anxiety, will be safe or effective.
In Ontario, a major ongoing study involving 10,000 patients is finding that, by matching drugs to gene types, doctors can predict which medication a patient will properly metabolize, and at what dose. It’s an approach that appears to be relieving symptoms, reducing side effects and curtailing hospital admissions.
Study leader James Kennedy, who is a professor of psychiatry at U of T and head of the Tanenbaum Centre of Pharmacogenetics at the Centre for Addiction and Mental Health, believes the final results will persuade the Ontario government to cover pharmacogenetic testing within two years, and that other provinces will follow.
“It’s still a long time to wait,” he says, because it’s not just the system that has to change, but doctors, too. “Physicians are pretty conservative for all kinds of new interventions, and this is a revolutionary change in the process a doctor uses to decide on a medication.
“Yet, the more this testing is delayed, suffering and unnecessary health-care costs are mounting up.”
Drug use in Canada at a glance

TOP FIVE PRESCRIPTION MEDICATIONS USED BY CANADIANS, AGES 25 TO 79
2007 to 2011, thousands of prescriptions
MALES
Lipid-modifying
agents, plain
1,697.60
Ace inhibitors, plain
978.1
Drugs for peptic
ulcer and GORD*
836.3
Beta-blocking agents
730.3
Other analgesics and
antipyretics
677.7
FEMALES
Antidepressants
1,514.00
Lipid-modifying
agents, plain
1,205.20
Thyroid
1,082.50
Drugs for peptic
ulcer and GORD*
1,011.80
Ace inhibitors, plain
659.6
*Gastroesophageal-reflux disease
THE GLOBE AND MAIL, SOURCE: STATISTICS CANADA

TOP FIVE PRESCRIPTION MEDICATIONS USED BY CANADIANS, AGES 25 TO 79
2007 to 2011, thousands of prescriptions
MALES
Lipid-modifying
agents, plain
1,697.60
Ace inhibitors, plain
978.1
Drugs for peptic
ulcer and GORD*
836.3
Beta-blocking agents
730.3
Other analgesics and
antipyretics
677.7
FEMALES
Antidepressants
1,514.00
Lipid-modifying
agents, plain
1,205.20
Thyroid
1,082.50
Drugs for peptic
ulcer and GORD*
1,011.80
Ace inhibitors, plain
659.6
*Gastroesophageal-reflux disease
THE GLOBE AND MAIL, SOURCE: STATISTICS CANADA

TOP FIVE PRESCRIPTION MEDICATIONS USED BY CANADIANS, AGES 25 TO 79
2007 to 2011, thousands of prescriptions
MALES
Lipid-modifying agents, plain
1,697.60
Ace inhibitors, plain
978.1
Drugs for peptic ulcer and GORD*
836.3
Beta-blocking agents
730.3
Other analgesics and antipyretics
677.7
FEMALES
Antidepressants
1,514.00
Lipid-modifying agents, plain
1,205.20
Thyroid
1,082.50
Drugs for peptic ulcer and GORD*
1,011.80
Ace inhibitors, plain
659.6
*Gastroesophageal-reflux disease
THE GLOBE AND MAIL, SOURCE: STATISTICS CANADA

TOP TWO PRESCRIPTION MEDICATIONS USED BY CANADIANS, AGES 6 TO 24
2007 to 2011, thousands of prescriptions
MALES
ADHD psychostimulants
and nootropics
146.2
Antidepressants
67.7
FEMALES
Systemic-use hormonal
contraceptives
494.3
280.3
Antidepressants
THE GLOBE AND MAIL, SOURCE: STATISTICS CANADA

TOP TWO PRESCRIPTION MEDICATIONS USED BY CANADIANS, AGES 6 TO 24
2007 to 2011, thousands of prescriptions
MALES
ADHD psychostimulants
and nootropics
146.2
Antidepressants
67.7
FEMALES
Systemic-use hormonal
contraceptives
494.3
280.3
Antidepressants
THE GLOBE AND MAIL, SOURCE: STATISTICS CANADA

TOP TWO PRESCRIPTION MEDICATIONS USED BY CANADIANS, AGES 6 TO 24
2007 to 2011, thousands of prescriptions
MALES
ADHD psychostimulants
and nootropics
146.2
Antidepressants
67.7
FEMALES
Systemic-use hormonal
contraceptives
494.3
280.3
Antidepressants
THE GLOBE AND MAIL, SOURCE: STATISTICS CANADA
‘Black box’ warnings and a pushback from marketers
While working at U of T in the 1950s, scientist Werner Kalow coined the term pharmacogenetics. He had been investigating why nasty, and sometimes fatal, drug reactions seemed to run in families. Toiling over test tubes and proteins extracted from the ground-up livers of animals, he discovered enzymes that break down drugs in the human liver – and realized that people inherit different levels of them.
Eventually, after it became possible to read DNA, scientists learned that these liver enzymes are made by a distinct family of genes. Known for short as CYP (pronounced “sip”) genes, about a half-dozen of them form the backbone of current pharmacogenetic tests, because they’re involved in metabolizing most of the drugs on the market. They convert certain medicines into their active form, make some drugs more or less toxic, and also break them down at different speeds: rapidly, moderately, slowly or, in some cases, not at all.
Every human carries CYP genes, but as with hair or skin colour, people inherit different versions of them. “Our liver enzymes evolved to protect us from poisonous plants that grew around [our ancestral populations] and what they ate to survive,” Dr. Kennedy explains, “and this has led to differences in how we metabolize drugs.”
Africans, for instance, have more diverse liver enzymes than do Asians or Caucasians, because their ancestors were exposed to a wider range of plants, and as the world’s oldest ancestral group, their genes have had more time to evolve. The result, says Dr. Kennedy, is that people of African descent often require higher drug doses, because their wider variety of enzymes tend to break down and eliminate medications more quickly. Asians, meanwhile, tend to require lower drug doses than do Caucasians to control certain conditions, such as high cholesterol, blood clotting and hypertension.

HOW METABOLIZER STATUS BREAKS DOWN,
WORLDWIDE, IN TWO MAJOR GENES
CYP2D6
Per-cent range of patients, by metabolizer status
0
20
40
60
80
100%
Ultrarapid
Normal
Intermediate
Poor
CYP2C19
Per-cent range of patients, by metabolizer status
0
20
40
60
80
100%
Ultrarapid
Rapid
Normal
Intermediate
Poor
THE GLOBE AND MAIL, SOURCE: NATIONAL CENTER
FOR BIOTECHNOLOGY INFORMATION

HOW METABOLIZER STATUS BREAKS DOWN,
WORLDWIDE, IN TWO MAJOR GENES
CYP2D6
Per-cent range of patients, by metabolizer status
0
20
40
60
80
100%
Ultrarapid
Normal
Intermediate
Poor
CYP2C19
Per-cent range of patients, by metabolizer status
0
20
40
60
80
100%
Ultrarapid
Rapid
Normal
Intermediate
Poor
THE GLOBE AND MAIL, SOURCE: NATIONAL CENTER
FOR BIOTECHNOLOGY INFORMATION

HOW METABOLIZER STATUS BREAKS DOWN,
WORLDWIDE, IN TWO MAJOR GENES
CYP2D6
Per-cent range of patients, by metabolizer status
0
20
40
60
80
100%
Ultrarapid
Normal
Intermediate
Poor
CYP2C19
Per-cent range of patients, by metabolizer status
0
20
40
60
80
100%
Ultrarapid
Rapid
Normal
Intermediate
Poor
THE GLOBE AND MAIL, SOURCE: NATIONAL CENTER FOR BIOTECHNOLOGY INFORMATION
But race is a crude and imperfect proxy for the true genetic picture: People of the same ethnicity can have very different genes. Yet, before the decoding of DNA got cheap, no company had much interest or financial incentive to develop tests to tell people how their genes metabolize drugs. “The knowledge around these individual genes,” says Dr. Kennedy, “was not patented.”
The consequences of ignorance, however, can be tragic.
In 2007, doctors at Sick Kids reported on the case of a newborn who died of a morphine overdose. Later genetic testing showed that the infant’s mother, who had been prescribed small doses of codeine following a painful childbirth, was an ultrarapid metabolizer whose body converted the drug into morphine so quickly that it became a lethal dose in the breast milk her baby ingested.
The case was key in prompting drug regulators to add a “black box” warning that codeine may cause breathing difficulties and death in patients who are ultrarapid metabolizers. Yet, as recently as 2012, doctors reported in the journal Pediatrics that children have continued to die – due to their own rapid metabolism of codeine – including a four-year-old First Nations boy prescribed the drug after having his tonsils and adenoids removed in Northern Ontario.
Still, even as it became faster and less expensive to sequence DNA, pharmaceutical companies were not keen to promote genetic tests that would limit the number of people who could take their drugs. Several times over the past 20 years, Dr. Kennedy says he has tried to convince them: “I’d tell them that [using genetic tests], I could predict the 70 per cent of people who would respond well to their medication.” But while company scientists wanted to hear more, the response was always the same from marketing executives – even after Dr. Kennedy warned that the drugs could fail or harm someone with mismatched genes: “They’d say, ‘If you eliminate 30 per cent of our market … we lose money on this drug and it would never be developed further.’”

Dr. Jim Kennedy of CAMH has been met with skepticism from pharmaceutical companies about the benefits of genetic tests.William Suarez
Both the U.S. FDA and Health Canada now recommend that drug companies trying to bring a new medication to market should include information about gene variants that could render the drug ineffective or dangerous in some patients. Neither regulatory agency makes it mandatory, but that, says Dr. Kennedy, could change with the outcome of a major lawsuit under way in Hawaii.
In 2014, that state-filed suit against Bristol-Myers Squibb and Sanofi-Aventis for allegedly marketing a drug the companies knew would not work for a major portion of the Hawaiian population. Plavix, or clopidogrel, is prescribed to prevent blood clots after someone has a heart attack or stroke, but native Hawaiians, says Dr. Kennedy, were dying at a higher rate while taking it.
Hawaii’s state attorney has said that 38 to 79 per cent of Pacific Islanders carry gene variants that hamper their ability to convert the drug to its active form. The FDA has since stamped a black-box warning on the drug stating that poor metabolizers who take it may remain at risk for cardiovascular death. “If Hawaii wins, it will cause the FDA to mandate that genetic-variant data should be provided and [drug companies] will have to do their trials in all different populations,” says Dr. Kennedy. “The lawsuit is going to be a pivotal point in the history of the field.”
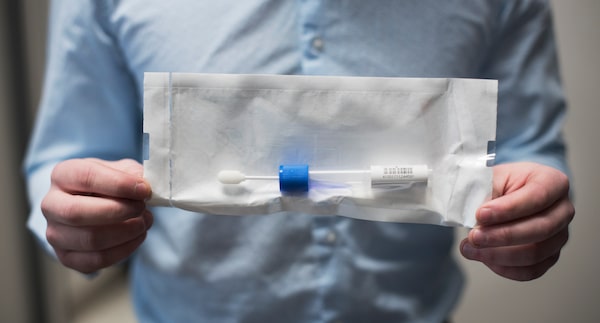
Richard Procunier holds a test kit from OneOme, a Minnesota-based company, that looks at markers in 22 genes and predicts a patient’s tolerance to more than 340 medications.Fred Lum/The Globe and Mail
Decades of puzzling reactions, finally understood
After he learned about pharmacogenetic tests in school, Mr. Procunier assumed they hadn’t been widely adopted because older doctors and pharmacists knew little about them. But the pharmacy he and a colleague opened in his hometown of North Bay happens to operate alongside a medical clinic also staffed by young doctors – and everyone was game to incorporate pharmacogenetics into their practices.
The challenge, he says, was finding a lab to run these specialized tests. The public-health system has few of them. While some major hospitals run genetic tests to predict responses to a few powerful medications to treat, say, HIV or cancer, especially in children – where the wrong drug or dose can be lethal – they rarely do it for other patients.
His search eventually led him to OneOme, a Minnesota company that offers a test developed at the Mayo Clinic. Used in private health-care plans and, according to the company website, many hospitals, the test looks at markers in 22 genes and predicts a patient’s tolerance to more than 340 medications. Mr. Procunier now sells it, along with his counselling services, for $349, and in contrast to the unknown quality of testing kits consumers can purchase online, he feels it’s “very comprehensive and evidence-based.” OneOme follows guidelines set by the international research groups that decide when there is sufficient evidence that a certain gene type can affect a person’s response to a specific drug.

The OneOme test kit is 'very comprehensive and evidence-based,' Mr. Procunier says.Fred Lum/The Globe and Mail
Mr. Procunier, who took extra training at the University of Florida to interpret results for customers and doctors, stresses that the test is only one of many factors that should guide therapy. Still, he concedes, because “this type of testing wasn’t very well known in North Bay, it caused a bit of a buzz.”
Among those surprised to read about the drugstore launch party in the local news was a 74-year-old woman who, for privacy reasons, has asked The Globe not to use her name. The woman knew about DNA tests that try to reveal ancestry or disease risks. But she’d never heard that a genetic test could tell her how her body might react to medicines – which, for 30 years, has been quite badly.
“I was very interested, anxious even, in having the test done. My husband takes 10 medications a day, throws them back with a glass of wine, and he’s just fine. But that’s never been my experience.” All of which compounds her general fears of going to the doctor: “Older women like me, we’re scared of doctors and hairdressers,” she says. “The hairdresser might find a bald spot, you don’t know what the doctors might find.”
In the 1980s, what they found was pelvic cancer, and she was sent for treatment to Toronto, where doctors gave her haloperidol, a potent tranquilizer, to calm her nerves. She woke that night in a fit of frightening hallucinations. By morning, her muscles had grown so stiff that she couldn’t bend her limbs, which stoked panic and anxiety that kept her off work for weeks afterward.
Later, when the dentist gave her an antibiotic to stave off infection after a tooth extraction, she broke out in a full-body rash. She has also suffered nasty side effects in response to an anti-anxiety medication, and nerve damage that still lingers from a drug to treat a urinary-tract infection. Meanwhile, she says, the antidepressant she’s taken for years does nothing for her: “I’m still struggling.”
So, last fall, she booked a private appointment with Mr. Procunier, recounted her medication history, and quizzed him about what the test could tell her, and what it couldn’t. (It cannot, for instance, predict most allergic reactions, and can offer information only about drugs and gene variants that have been studied.) By the end of the session, she swabbed her inner cheek to gather a DNA sample, and Mr. Procunier mailed it to the Minnesota company for analysis.
A week later, the results came back, and with them, a realization: “All these things that had happened to me, it wasn’t my fault, it wasn’t in my head,” she says, “It was in my DNA.”
The test found that she carries one gene that makes her a poor metabolizer of some drugs, including haloperidol, increasing her risk of toxicity. By contrast, a variant in another gene makes her a rapid metabolizer of some other medications, which might explain why her antidepressant doesn’t work, says Mr. Procunier: Her body could be breaking it down too quickly.
The woman’s pharmacogenetic report runs 10 pages, and though one section sorts the results under simple traffic-light icons – green for drugs that she can likely tolerate, yellow for those that should be used with caution, red for those likely to cause problems – “it isn’t,” she says, “something you can pick up and understand immediately.”
But Mr. Procunier walked her through it, and plans to explain it to her doctor. Which, she says, is a big relief: Her physician is not part of the Northern Shores clinic and she’s apprehensive about how he will react to a medical test she ordered herself. Still, she says, with no other way for a doctor to know how a patient might react to a new drug, or a particular dose, “Surely it would benefit all concerned if a patient’s DNA profile is reviewed.”

'Health care is lagging a little bit behind' on genetic testing, creating a gap where unregulated testing companies that do substandard work can thrive, says Iris Cohn, pharmacogenetics adviser at Sick Kids Hospital in Toronto.Robert Teteruck
One ‘great loophole’ and a market that will not wait
In the Personal Genome Project, based at Sick Kids and U of T’s McLaughlin Centre, all 56 of the inaugural participants learned something medically actionable about their genetic response to drugs. In one case, the slow metabolism profile of a 28-year-old woman was found to be so worrisome that researchers immediately warned her about her increased risks of drug toxicity.
Iris Cohn, the pharmacogenetics adviser at Sick Kids who leads the project’s analysis of gene and drug interactions, says the results demonstrate the value of pharmacogenomics, and why patients are seeking out the tests: “because health care is lagging a little bit behind.”
Published last month in the Canadian Medical Association Journal, the study included results that hit close to home for Ms. Cohn. Her husband, Ronald Cohn, Sick Kids’ chief of pediatrics, was among the participants, and he had previously been prescribed Percocet to treat acute back pain. A mix of oxycodone and Tylenol, the drug is a fast-acting opioid. Yet, a while after he took it, she says, “He looked at me and said, ‘Okay, when is this supposed to help?’ ”
Only after his genome was sequenced did Ms. Cohn learn that her husband carries a CYP gene that makes him unable to convert oxycodone into its active form of morphine. Armed with that knowledge, she says, he now knows that he can never turn to any opioid for pain relief – “He would have to go directly to morphine.”
Pharmacists are a logical choice to interpret these kinds of results, says Ms. Cohn. What worries her are the online companies selling mail-order tests. “I don’t know who is testing, what they are looking at and who is interpreting it.”
Health Canada regulates only those companies that market their test kits for medical purposes, and it has licensed 20 such companies to date. But if – as most companies stipulate – the test kit is sold for “research purposes only,” no licence is required.
“This is the great loophole,” says Ms. Cohn, “Hundreds of pharmacogenetic testing companies are popping up, but they are not regulated.” And as she discovered, their work can be substandard. A few participants in the Personal Genome Project were found to have different pharmacogenetic results than those a private company had previously reported. But the firm “didn’t even deny” its results were incorrect when she called to discuss the discrepancy.
“The good thing about people going with direct-to-consumer companies is that it pushes the field forward. The negative thing is that it pushes this information into clinical care, but you don’t know if you can trust the information,” says Ms. Cohn, “That’s the conundrum we’re in.”
Bruce Carleton, chair of clinical pharmacology in the University of British Columbia’s pediatrics department, believes pharmacogenetic tests “have a lot of potential to help people make more informed decisions.” But, he says, dubious tests do have the potential to cause real harm: “If they say, ’Don’t use this drug because of the risk of adverse events, well, [how big] is the risk? I worry about people not getting the therapy they need, because they’re worried about an adverse effect that may have a very low likelihood of occurring.” Dr. Carleton, who also heads the Canadian Pharmacogenomics Network for Drug Safety, says patients should discuss these kinds of test results with their doctors in the context of medication they need.
Studies have found, however, that while doctors are interested in pharmacogenetics, they’re reluctant to order such tests or to try to interpret them. “This type of information is not taught well in medical schools, if at all,” says Richard Kim, a clinical pharmacologist at the London Health Sciences Centre.
And interpreting these results is not simple, he adds. Many drugs are broken down by more than one gene, so the interplay of two or three can shape a drug response. And several factors beyond genes can play a role: age, weight, other health conditions, other drugs being taken, smoking, drinking alcohol and general diet.
Yet, says Dr. Kim, who’s also a scientist with the Lawson Health Research Institute, many patients who take the consumer tests believe that the results tell the whole story, and that can lead to unnecessary health-care visits. He says he has had his share of anxious patients turn up with their results at the specialized pharmacogenetic testing clinic he runs: “But then I look at their results and I go, ‘Wow, this is pretty much an average person.’ ”
Dr. Richard Kim works in his lab at University Hospital in London, Ont.GEOFF ROBINS/The Globe and Mail
Whether the health-care system is ready or not, some proponents argue that people should know their drug metabolism status as they do their blood type. Corey Nislow, associate professor of pharmaceutical sciences at UBC, says the current tests may for now be “the bronze standard” as the science expands, but he argues that “every self-aware, genetically savvy professional I know, knows their metabolism status and they should – everyone should.”
In fact, because Dr. Nislow knows his drug-gene profile, doctors knew to give him only half the regular dose of a blood thinner when he underwent a recent surgery. “It makes me feel guilty,” he says, “that this is not universally available.”
Dr. Nislow is leading a study with the BC Pharmacy Association exploring how best to make testing widely available through specially trained pharmacists at community drugstores. Although the research is ongoing, the non-profit Pharmacy Association last year launched a for-profit spin-off called RxOme. It has partnered with Australian firm myDNA Life, which sells a pharmacogenetics test through drugstores in that country and now, through more than 100 Canadian pharmacies. RxOme reports that “several hundred Canadians” have so far taken the test, with their DNA sent to Australia for analysis.
“This is a case where the market is not waiting,” says Dr. Nislow, who is not involved with the new venture. “It’s about saying, let’s just test what we can test for now without worrying about the long-term question of how we can build that into primary care.”
Governments, he says, have in part been hesitant to incorporate a new test, as they tend to see it as an added cost. But he calls this “obscenely short-sighted,” since the one-time cost can be “amortized” over a lifetime.
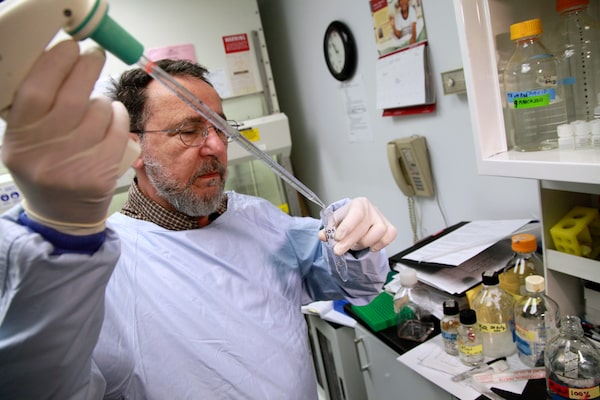
David Sibony, research analyst on CAMH’s IMPACT study team, extracts DNA in the lab. Some 3,000 doctors are testing the genes of 10,000 patients to explore the merits of gene-based drug treatment.
A broad new study and insurer curiosity
Eight years ago, Dr. Kennedy at CAMH began working with the Ontario government to design a study that might prove that pharmacogenetics could prevent patient suffering and save the system money in mental-health treatment. Launched in 2012, the ongoing IMPACT study involves 3,000 doctors testing the drug-response genes of 10,000 patients and comparing their progress to people with matching characteristics and conditions who did not receive gene-guided treatment.
Along with capturing the genetic diversity of Toronto, researchers turned a tractor-trailer into a mobile lab and spent five years on the road recruiting patients in Indigenous communities, agricultural centres and areas hard hit by job losses.
The test looks at variants in eight major CYP metabolism genes and two brain genes involved in handling serotonin, the mood neurochemical targeted by a popular class of antidepressants and anti-anxiety drugs. Results are returned to doctors in a user-friendly report that predicts how well a patient will tolerate 38 medications approved to treat mental illness.
In the United States, a 2013 study found that people taking antidepressants matched to their genes had 69-per-cent fewer total health-care visits and a quarter the disability claims of people who took an antidepressant mismatched to their genetic profiles. Dr. Kennedy believes that the IMPACT results, expected in 2019, will also show the test is a dramatic improvement over the current trial-and-error process, which can drag on for months.
“Patients really suffer,” he says, “They lose confidence in their meds, in their doctor, they fall out of care, they get worse and end up hospitalized, and it leads to very bad outcomes, including suicide.”
Mental illness may be the catalyst that drives pharmacogenetics into health care, but Dr. Kennedy suspects that savvy doctors will ultimately use the metabolism test results to prescribe a variety of other medications.
But for now, mental illness is also the prime focus of the insurance industry’s investigations into pharmacogenetics. Last August, for instance, Sun Life gave clients already on an approved mental health-related disability claim the option to join the IMPACT study. In December, Great-West Life launched a pilot project with a private pharmacogenetics testing company to see if it can help clients with chronic pain and mental-health conditions. And Manulife is rolling out a pharmacogenetics study to guide treatment for depression, anxiety and chronic pain.
“It’s certainly significant to us to see the three largest insurers in Canada all running pilot programs at the same time,” says Valerie Charlebois, a health and benefits expert at Mercer, an international human-resources consulting firm.
Ms. Charlebois says interest is also growing among employers. Absenteeism costs the Canadian economy more than $16-billion annually, and each week, half a million workers are absent due to mental illness, which is the leading cause of disability. With the potential of gene-guided prescribing, she explains, “employers could see reduced disability costs and reduced drug-plan costs, and potentially return their healthy employees to work.”
The main concern centres on privacy, she says: Human-resources managers feel that “employees would be worried that employers would have access to their genetic information.” Canada’s new genetic anti-discrimination law, Bill S-201, makes it illegal for businesses to ask their workers to take a genetic test or to share the results of any genetic test they take. So employers would have to work around that, possibly offering the test as an option through a testing company at the time of a disability claim.
To further her research in the field, Ms. Charlebois recently took two pharmacogenetic tests herself – and discovered that she is a poor metabolizer in one of her major CYP genes, putting her at increased risk of toxicity and side effects in response to several medications.
“I don’t have use for these medications right now, but it’s something I would share with my physician,” she says, “It’s a powerful tool.” And when she showed her test results to colleagues back at the office, everyone had the same reaction: “Oh, I want to do that.”
Carolyn Abraham is a science journalist and the author of The Juggler’s Children: A Journey into Family, Legend and the Genes that Bind Us, a bestselling memoir that explored the power of DNA tests to solve her own family mysteries, and which was a finalist for the Governor-General’s Literary Award for Nonfiction.
Genomics: More from The Globe and Mail
Cracks in the code: Why mapping your DNA may be less reliable than you think